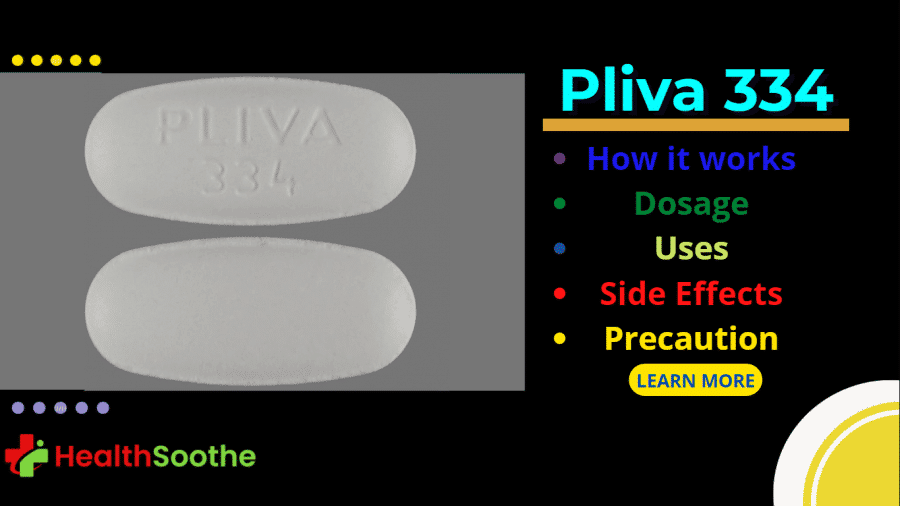
Have you ever had bacterial infections like acne, pneumonia, tuberculosis, upper respiratory tract infection (although usually viral), gastritis, food poisoning, eye infections, and others?
[ninja_tables id=”73025″]
If you did, then you must have needed ways or treatments to do away with this infection. Various treatments are available to treat bacterial infections, and antibiotics are among them.
Antibiotics are usually the first go-to treatments that many people typically use to treat bacterial infections, and Pliva 334 is one of the antibiotic medications available that is used to treat bacterial infections.
If you are thinking of using Pliva 334 for your bacterial infection, and you wanna know whether this medication is effective or suitable for you, then you are at the right place and site – Healthsoothe.
Or have Pliva 334 been recommended or prescribed for you, and before you use this antibiotic, you want to know its benefits, how it works, its side effects, precautions/warnings, and much more, then don’t worry because this article will answer all those questions and more that you might have Pliva 334.
This article will discuss how Pliva 334 works, its dosage, uses, side effects, precautions, and interactions.
Read on.
Pliva 334 – What is it?

It is a drug that belongs to drug classes; antibiotics (miscellaneous), nitroimidazole antimicrobials, and amebicides, and is majorly used in the treatment of bacterial infections and specific kinds of infections.
Amebicides are agents that destroy or kill amebae (a parasitic species), while miscellaneous antibiotics are antibiotics that are the only agent available in their class.
This means that they are unique in their action and not comparable to other antibiotics, although their spectrum of activity or certain side effects may be similar to other antibiotics.
And nitroimidazole antimicrobials are substances that work toward stopping the growth of bacteria.
So Pliva 334 belongs to both classes, and so works as both.
Pliva 334 drug summary details
- Drug class: Antibiotics (miscellaneous), nitroimidazole antimicrobials, and amebicides
- Active ingredients: Metronidazole
- Inactive ingredients: silicon dioxide, cottonseed oil (hydrogenated), and microcrystalline cellulose.
- Size: 15.00 – 20mm
- Pregnancy category: The level is B which means no proven risk is observed.
- Strength: Commonly 500 mg (though this could vary depending on the form or does you want)
- Color: White
- Forms: Capsules (pills), intravenous infusion (IV), compounding powder, intravenous powder for injection, intravenous solution, oral capsule, oral suspension, oral tablet, oral tablet extended release.
- Shape: Oval or elliptical
- CSA Schedule: Not controlled
- Supplier/Labeler: Teva Pharmaceuticals, U.S.A.
- National Drug Code (NDC): 50111-0334
- Drug imprint: PLIVA 334
- Manufacturer: Teva Pharmaceuticals, U.S.A.
- Availability: Prescription only
How does Pliva 334 work?
The active ingredient of pliva 334 is metronidazole, which works to hamper the growth of bacteria.
Pliva 334 belongs to the following drug classes;
- Amebicides: These are agents that destroy or kill amebae (a parasitic species).
- Antibiotics (miscellaneous): Miscellaneous antibiotics are antibiotics that are the only agent available in their class. This means that they are unique in their action and not comparable to other antibiotics, although their spectrum of activity or certain side effects may be similar to other antibiotics.
- Nitroimidazole antimicrobials: These are substances that work towards stopping the growth of bacteria. They work by stopping the growth of bacteria by using nitroimidazoles which are bactericidal through toxic metabolites which cause DNA strand breakage, thereby hampering the growth and thereby killing it.
Antibiotics like Pliva 334 will not work for colds, flu, or other viral infections.
How should I use PLIVA 334?
This is how to use the IV form of Pliva 334;
- IV: Individualize dosage, rate of administration, and duration of therapy (depend on the indication for use; patient age, weight, clinical condition, concomitant therapy; clinical and laboratory response of the patient to therapy).
- IV: Administer via slow IV drip infusion only, either as a continuous or intermittent infusion; do not use flexible containers in series connections.
- IV: If used with a primary IV fluid system, stop the primary solution during infusion of this drug.
- IV: Do not use equipment containing aluminum (e.g., needles, cannulae) that would come in contact with drug solution (precipitates may form).
IV compatibility:
- Incompatible (includes but is not limited to): Aztreonam, cefamandole nafate, cefoxitin, penicillin G
- Additives should not be introduced unless compatibility is known.
Most anaerobic severe infections are treated with IV therapy initially; may switch to oral therapy when conditions warrant, based on the severity of the disease and patient response to IV therapy
In general, the duration of therapy is 7 to 10 days; infections of the bone and joint, lower respiratory tract, and endocardium may require longer therapy.
Extended-release tablets: Do not split, crush, or chew; administer at least 1 hour before or 2 hours after meals (under fasting conditions).
Take Pliva 334 exactly as prescribed by your doctor. Follow all directions on your prescription label and read all medication guides or instruction sheets.
Pliva 334 oral is taken by mouth. The injection is given as an infusion into a vein. A healthcare provider will give you the injection if you are unable to take the medicine by mouth.
Shake the oral suspension (liquid). Measure a dose with the supplied measuring device (not a kitchen spoon).
Swallow the extended-release tablet whole and do not crush, chew, or break it.
If you are treating a vaginal infection, your sexual partner may also need to take Pliva 334 so you don’t become infected again.
Pliva 334 is usually given for up to 10 days in a row. You may need to repeat this dosage several weeks later.
Keep using this medicine even if your symptoms quickly improve. Skipping doses could make your infection resistant to medication. This medicine will not treat viral infection (flu or a common cold).
Pliva 334 will not treat a vaginal yeast infection. You may even develop a new vaginal yeast infection, which may need to be treated with antifungal medication. Tell your doctor if you have symptoms such as itching or discharge during or after treatment with this medicine.
Do not share this medicine with another person, even if they have the same symptoms you have.
This medicine can affect the results of certain medical tests. Tell any doctor who treats you that you are using this medicine.
Store at room temperature away from moisture and heat.
Dosing instructions for PLIVA 334
Usual Adult Dose for Bacterial Infection
- Loading dose: 15 mg/kg IV once as a single dose
- Maintenance dose: 7.5 mg/kg IV or orally every 6 hours
- Maximum dose: 4 g/day
- The usual duration of therapy: 7 to 10 days
Comments:
- Most anaerobic severe infections are treated with IV therapy initially.
- Loading and maintenance doses should be infused IV over 1 hour.
- The first IV maintenance dose should be started 6 hours after the start of the loading dose.
- May switch from IV to oral therapy when conditions warrant, based on the severity of disease and patient response to IV therapy
- Infections of the lower respiratory tract and endocardium may require longer treatment.
Uses: For the treatment of serious infections due to susceptible anaerobic bacteria including:
- Gynecologic infections (including endometritis, endometritis, tubo-ovarian abscess, postsurgical vaginal cuff infection) due to Bacteroides species (including Bacteroides fragilis group [B fragilis, B distasonis, B ovatus, B thetaiotaomicron, B. vulgatus]), Clostridium species, Peptococcus species, Peptostreptococcus species, or Fusobacterium species
- Bacterial septicemia due to Bacteroides species (including B fragilis group) or Clostridium species
- CNS infections (including meningitis, and brain abscess) due to Bacteroides species (including B fragilis group)
- Lower respiratory tract infections (including pneumonia, empyema, lung abscess) due to Bacteroides species (including B fragilis group)
- Endocarditis due to Bacteroides species (including B fragilis group)
Usual Adult Dose for Intra-abdominal Infection
- Loading dose: 15 mg/kg IV once as a single dose
- Maintenance dose: 7.5 mg/kg IV or orally every 6 hours
- Maximum dose: 4 g/day
- The usual duration of therapy: 7 to 10 days
Comments:
- Most anaerobic severe infections are treated with IV therapy initially.
- Loading and maintenance doses should be infused IV over 1 hour.
- The first IV maintenance dose should be started 6 hours after the start of the loading dose.
- May switch from IV to oral therapy when conditions warrant, based on the severity of disease and patient response to IV therapy
Uses: For the treatment of serious intra-abdominal infections (including peritonitis, intra-abdominal abscess, liver abscess) due to susceptible Bacteroides species (including B fragilis group), Clostridium species, Eubacterium species, Peptococcus species, or Peptostreptococcus species
Infectious Diseases Society of America (IDSA) and Surgical Infection Society (SIS) Recommendations: 500 mg IV every 8 to 12 hours or 1500 mg IV every 24 hours
Comments:
- With other antibacterial agents, recommended as a combination regimen for empiric treatment of complicated intra-abdominal infection
- Current guidelines should be consulted for additional information.
Usual Adult Dose for Peritonitis
- Loading dose: 15 mg/kg IV once as a single dose
- Maintenance dose: 7.5 mg/kg IV or orally every 6 hours
- Maximum dose: 4 g/day
- The usual duration of therapy: 7 to 10 days
Comments:
- Most anaerobic severe infections are treated with IV therapy initially.
- Loading and maintenance doses should be infused IV over 1 hour.
- The first IV maintenance dose should be started 6 hours after the start of the loading dose.
- May switch from IV to oral therapy when conditions warrant, based on the severity of disease and patient response to IV therapy
Uses: For the treatment of serious intra-abdominal infections (including peritonitis, intra-abdominal abscess, liver abscess) due to susceptible Bacteroides species (including B fragilis group), Clostridium species, Eubacterium species, Peptococcus species, or Peptostreptococcus species.
Infectious Diseases Society of America (IDSA) and Surgical Infection Society (SIS) Recommendations: 500 mg IV every 8 to 12 hours or 1500 mg IV every 24 hours
Comments:
- With other antibacterial agents, recommended as a combination regimen for empiric treatment of complicated intra-abdominal infection
- Current guidelines should be consulted for additional information.
Usual Adult Dose for Liver Abscess
- Loading dose: 15 mg/kg IV once as a single dose
- Maintenance dose: 7.5 mg/kg IV or orally every 6 hours
- Maximum dose: 4 g/day
- The usual duration of therapy: 7 to 10 days
Comments:
- Most anaerobic severe infections are treated with IV therapy initially.
- Loading and maintenance doses should be infused IV over 1 hour.
- The first IV maintenance dose should be started 6 hours after the start of the loading dose.
- May switch from IV to oral therapy when conditions warrant, based on the severity of disease and patient response to IV therapy
Uses: For the treatment of serious intra-abdominal infections (including peritonitis, intra-abdominal abscess, liver abscess) due to susceptible Bacteroides species (including B fragilis group), Clostridium species, Eubacterium species, Peptococcus species, or Peptostreptococcus species
Infectious Diseases Society of America (IDSA) and Surgical Infection Society (SIS) Recommendations: 500 mg IV every 8 to 12 hours or 1500 mg IV every 24 hours
Comments:
- With other antibacterial agents, recommended as a combination regimen for empiric treatment of complicated intra-abdominal infection
- Current guidelines should be consulted for additional information.
Usual Adult Dose for Joint Infection
- Loading dose: 15 mg/kg IV once as a single dose
- Maintenance dose: 7.5 mg/kg IV or orally every 6 hours
- Maximum dose: 4 g/day
- The usual duration of therapy: 7 to 10 days
Comments:
- Most anaerobic severe infections are treated with IV therapy initially.
- Loading and maintenance doses should be infused IV over 1 hour.
- The first IV maintenance dose should be started 6 hours after the start of the loading dose.
- May switch from IV to oral therapy when conditions warrant, based on the severity of disease and patient response to IV therapy
- Infections of the bone and joint may require longer treatment.
Uses: As adjunctive therapy for the treatment of bone and joint infections due to Bacteroides species (including B fragilis group)
IDSA Recommendations: 500 mg orally 3 to 4 times a day
Comments:
- This drug can be used in the initial treatment of native vertebral osteomyelitis due to Bacteroides species and other susceptible aerobes.
- Current guidelines should be consulted for additional information.
Usual Adult Dose for Osteomyelitis
- Loading dose: 15 mg/kg IV once as a single dose
- Maintenance dose: 7.5 mg/kg IV or orally every 6 hours
- Maximum dose: 4 g/day
- The usual duration of therapy: 7 to 10 days
Comments:
- Most anaerobic severe infections are treated with IV therapy initially.
- Loading and maintenance doses should be infused IV over 1 hour.
- The first IV maintenance dose should be started 6 hours after the start of the loading dose.
- May switch from IV to oral therapy when conditions warrant, based on the severity of disease and patient response to IV therapy
- Infections of the bone and joint may require longer treatment.
Uses: As adjunctive therapy for the treatment of bone and joint infections due to Bacteroides species (including B fragilis group)
IDSA Recommendations: 500 mg orally 3 to 4 times a day
Comments:
- This drug can be used in the initial treatment of native vertebral osteomyelitis due to Bacteroides species and other susceptible aerobes.
- Current guidelines should be consulted for additional information.
Usual Adult Dose for Skin or Soft Tissue Infection
- Loading dose: 15 mg/kg IV once as a single dose
- Maintenance dose: 7.5 mg/kg IV or orally every 6 hours
- Maximum dose: 4 g/day
- The usual duration of therapy: 7 to 10 days
Comments:
- Most anaerobic severe infections are treated with IV therapy initially.
- Loading and maintenance doses should be infused IV over 1 hour.
- The first IV maintenance dose should be started 6 hours after the start of the loading dose.
- May switch from IV to oral therapy when conditions warrant, based on the severity of disease and patient response to IV therapy.
Use: For the treatment of serious skin and skin structure infections due to susceptible Bacteroides species (including B fragilis group), Clostridium species, Peptococcus species, Peptostreptococcus species, or Fusobacterium species
- IDSA Recommendations:
- Incisional surgical site infection: 500 mg IV every 8 hours
- Necrotizing infection: 500 mg IV every 6 hours
Infection after animal bite:
- IV: 500 mg IV every 8 hours
- Oral: 250 to 500 mg orally 3 times a day
Comments:
- With ceftriaxone, ciprofloxacin, or levofloxacin, recommended as a combination regimen for treatment of incisional surgical site infections after intestinal or genitourinary tract surgery
- With ceftriaxone, ciprofloxacin, or levofloxacin, recommended for treatment of incisional surgical site infections after surgery of axilla or perineum; coverage for methicillin-resistant S aureus (MRSA) may be needed.
- In combination with cefotaxime, recommended for the treatment of necrotizing infections of the skin, fascia, and muscle due to mixed infections
- Recommended for the treatment of infection after an animal bite; should consider that this drug has no activity against aerobes.
- Current guidelines should be consulted for additional information.
Usual Adult Dose for Skin and Structure Infection
- Loading dose: 15 mg/kg IV once as a single dose
- Maintenance dose: 7.5 mg/kg IV or orally every 6 hours
- Maximum dose: 4 g/day
- The usual duration of therapy: 7 to 10 days
Comments:
- Most anaerobic severe infections are treated with IV therapy initially.
- Loading and maintenance doses should be infused IV over 1 hour.
- The first IV maintenance dose should be started 6 hours after the start of the loading dose.
- May switch from IV to oral therapy when conditions warrant, based on severity of disease and patient response to IV therapy.
Use: For the treatment of serious skin and skin structure infections due to susceptible Bacteroides species (including B fragilis group), Clostridium species, Peptococcus species, Peptostreptococcus species, or Fusobacterium species
IDSA Recommendations:
- Incisional surgical site infection: 500 mg IV every 8 hours
- Necrotizing infection: 500 mg IV every 6 hours
Infection after an animal bite:
- IV: 500 mg IV every 8 hours
- Oral: 250 to 500 mg orally 3 times a day
Comments:
- Ceftriaxone, ciprofloxacin, or levofloxacin, are recommended as a combination regimen for treatment of incisional surgical site infections after intestinal or genitourinary tract surgery
- With ceftriaxone, ciprofloxacin, or levofloxacin, recommended for treatment of incisional surgical site infections after surgery of axilla or perineum; coverage for methicillin-resistant S aureus (MRSA) may be needed.
- In combination with cefotaxime, recommended for the treatment of necrotizing infections of the skin, fascia, and muscle due to mixed infections
- Recommended for the treatment of infection after animal bite; should consider that this drug has no activity against aerobes.
- Current guidelines should be consulted for additional information.
Usual Adult Dose for Wound Infection
- Loading dose: 15 mg/kg IV once as a single dose
- Maintenance dose: 7.5 mg/kg IV or orally every 6 hours
- Maximum dose: 4 g/day
Usual duration of therapy: 7 to 10 days
Comments:
- Most anaerobic severe infections are treated with IV therapy initially.
- Loading and maintenance doses should be infused IV over 1 hour.
- The first IV maintenance dose should be started 6 hours after the start of the loading dose.
- May switch from IV to oral therapy when conditions warrant, based on the severity of disease and patient response to IV therapy.
Use: For the treatment of serious skin and skin structure infections due to susceptible Bacteroides species (including B fragilis group), Clostridium species, Peptococcus species, Peptostreptococcus species, or Fusobacterium species
IDSA Recommendations:
- Incisional surgical site infection: 500 mg IV every 8 hours
- Necrotizing infection: 500 mg IV every 6 hours
Infection after animal bite:
- IV: 500 mg IV every 8 hours
- Oral: 250 to 500 mg orally 3 times a day
Comments:
- Ceftriaxone, ciprofloxacin, or levofloxacin, are recommended as a combination regimen for treatment of incisional surgical site infections after intestinal or genitourinary tract surgery
- With ceftriaxone, ciprofloxacin, or levofloxacin, recommended for treatment of incisional surgical site infections after surgery of axilla or perineum; coverage for methicillin-resistant S aureus (MRSA) may be needed.
- In combination with cefotaxime, it is recommended for the treatment of necrotizing infections of the skin, fascia, and muscle due to mixed infections.
- Recommended for the treatment of infection after an animal bite; should consider that this drug has no activity against aerobes.
- Current guidelines should be consulted for additional information.
Usual Adult Dose for Amebiasis
- Acute intestinal amebiasis: 750 mg orally 3 times a day for 5 to 10 days
- Amebic liver abscess: 500 or 750 mg orally 3 times a day for 5 to 10 days
Comments:
Aspiration or drainage of pus is still needed for amebic liver abscess.
Uses: For the treatment of acute intestinal amebiasis (acute amebic dysentery) and amebic liver abscess
Usual Adult Dose for Surgical Prophylaxis
- Initial preoperative dose: 15 mg/kg IV infused over 30 to 60 minutes and completed about 1 hour before surgery
- Followed by: 7.5 mg/kg IV infused over 30 to 60 minutes at 6 and 12 hours after the initial dose
Comments:
- Important to complete administration of the initial preoperative dose about 1 hour before surgery to achieve adequate drug levels in serum and tissue at the time of initial incision
- Important to administer this drug, if needed, at 6-hour intervals to maintain effective drug levels
- Prophylactic use should be limited to the day of surgery, following the above guidelines; if signs of infection, specimens for culture should be obtained to identify causative organism(s) so that appropriate therapy may be administered.
Use: For surgical prophylaxis, to prevent postoperative infection in contaminated/potentially contaminated colorectal surgery
IDSA, SHEA, American Society of Health-System Pharmacists (ASHP), and SIS Recommendations:
- Preoperative dose: 500 mg IV as a single dose
- Colorectal surgery prophylaxis: 1 g orally
Comments:
- IV: This drug should be started within 60 minutes before the surgical incision.
- IV: A single prophylactic dose is usually sufficient; if prophylaxis is continued postoperatively, the duration should be less than 24 hours.
- IV: Redosing may be needed for unusually long procedures.
- Oral: In most patients, mechanical bowel preparation combined with this oral drug plus oral neomycin should be administered in addition to IV prophylaxis; the oral regimen should be administered in 3 doses over about 10 hours, beginning after the mechanical bowel preparation, the afternoon, and evening before the procedure.
- Coadministration with other agents may be recommended, depending on the type of procedure.
- Current guidelines should be consulted for additional information.
Uses: Recommended for surgical prophylaxis for the following procedures:
- Appendectomy for uncomplicated appendicitis
- Biliary tract: Open procedure; elective, high-risk laparoscopic procedure
- Colorectal
- Head and neck: Clean-contaminated cancer surgery; other clean-contaminated procedures (excluding tonsillectomy, and functional endoscopic sinus procedures)
- Hysterectomy: Vaginal or abdominal
- Small intestine: Obstructed
- Urologic: Clean-contaminated
Usual Adult Dose for Trichomoniasis
- Capsules: 375 mg orally twice a day for 7 consecutive days
Tablets:
- One-day regimen: 2 g orally as a single dose or 1 g twice given on the same day
- Seven-day regimen: 250 mg orally 3 times a day for 7 consecutive days
Comments:
- Single-dose regimens can assure compliance, however, a 7-day course may minimize reinfection by protecting the patient long enough for the sexual contact to be treated.
- The dose regimen should be individualized in females and males; some patients may tolerate 1 regimen more than the other.
- The patient’s sexual partner(s) should also be evaluated/treated.
- If repeat courses are required, an interval of 4 to 6 weeks should elapse between courses and the presence of the trichomon may be reconfirmed by appropriate laboratory testing.
- Pregnant patients should not be treated during the first trimester; if alternative therapy was not adequate in a pregnant patient, the 1-day regimen is not recommended (results in higher serum levels that can reach fetal circulation).
- The trichomonas can interfere with the accurate assessment of abnormal cytological smears; additional smears should be performed after the parasite is eradicated.
- Whether to treat an asymptomatic male partner with a negative culture (or with no culture) is an individual decision; should consider that a woman may be reinfected if her sexual partner is not treated and that it is considerably difficult to isolate the organism from asymptomatic male carriers so negative smears and cultures cannot be relied upon
- The sexual partner should be treated with this drug if reinfection occurs.
Uses:
- Symptomatic trichomoniasis: For the treatment of Trichomonas vaginalis infection in females and males when confirmed by appropriate laboratory procedures (wet smears and/or cultures)
- Asymptomatic trichomoniasis: For the treatment of asymptomatic T vaginalis infection in females when associated with endocervicitis, cervicitis, or cervical erosion
- Treatment of asymptomatic sexual partners: For the simultaneous treatment of asymptomatic sexual partners of treated patients if T vaginalis is present to prevent reinfection of the partner
US CDC Recommendations: 2 g orally once as a single dose
- Alternative regimen: 500 mg orally twice a day for 7 days
- Trichomoniasis in HIV-infected women: 500 mg orally twice a day for 7 days
- Treatment failure with single-dose therapy and reinfection is excluded: 500 mg orally twice a day for 7 days
- For patients failing this regimen: 2 g orally once a day for 7 days should be considered
Comments:
- Sexual partner(s) should be treated simultaneously with the same dose; appropriate treatment of sexual partner(s) may increase reported cure rates.
- If treatment is considered, the recommended regimen in pregnant women is 2 g orally as a single dose; symptomatic women, at any stage of pregnancy, should be tested and considered for treatment.
- Current guidelines should be consulted for additional information.
- Usual Adult Dose for Bacterial Vaginosis
- Extended-release tablets: 750 mg orally once a day for 7 consecutive days
Use: For the treatment of bacterial vaginosis in nonpregnant women
US CDC Recommendations:
Immediate-release tablets: 500 mg orally twice a day for 7 days
Comments:
- Treatment is recommended for all women with symptoms.
- Current guidelines should be consulted for additional information.
Usual Adult Dose for Pseudomembranous Colitis
IDSA and Society for Healthcare Epidemiology of America (SHEA) Recommendations:
- The initial episode of nonsevere Clostridium difficile infection (CDI): 500 mg orally 3 times a day for 10 to 14 days
- The initial episode of fulminant CDI: 500 mg IV every 8 hours
Comments:
- Oral: Recommended as an alternative agent for an initial episode of nonsevere CDI
- IV: With vancomycin (oral or rectal), recommended for an initial episode of fulminant CDI, especially if ileus is present
- Fulminant CDI may be characterized by hypotension/shock, ileus, or megacolon.
- Current guidelines should be consulted for additional information.
Usual Adult Dose for Helicobacter pylori Infection
American College of Gastroenterology Recommendations
First-line Regimens for Helicobacter pylori Infection:
- Bismuth quadruple therapy: 250 mg orally 4 times a day or 500 mg orally 3 to 4 times a day
- Clarithromycin-based triple therapy: 500 mg orally 3 times a day
- Concomitant therapy, fluoroquinolone sequential therapy, hybrid therapy, or sequential therapy: 500 mg orally twice a day
Duration of Therapy:
- Bismuth quadruple therapy: 10 to 14 days
- Clarithromycin-based triple therapy: 14 days
- Concomitant therapy: 10 to 14 days
- Fluoroquinolone sequential therapy: 5 to 7 days
- Hybrid therapy: 7 days
- Sequential therapy: 5 to 7 days
Salvage Regimens for H pylori Infection:
- Bismuth quadruple therapy: 500 mg orally 3 to 4 times a day for 14 days
- Concomitant therapy: 500 mg orally 2 to 3 times a day for 10 to 14 days
Comments:
- Bismuth quadruple therapy: Consists of a proton pump inhibitor (PPI), bismuth, tetracycline, and a nitroimidazole
- Clarithromycin-based triple therapy: Consists of a PPI, clarithromycin, and (amoxicillin or this drug)
- Concomitant therapy: Consists of a PPI, clarithromycin, amoxicillin, and a nitroimidazole
- Fluoroquinolone sequential therapy: Consists of a PPI and amoxicillin initially followed by a PPI, a fluoroquinolone, and a nitroimidazole
- Hybrid therapy: Consists of a PPI and amoxicillin initially followed by a PPI, amoxicillin, clarithromycin, and a nitroimidazole
- Sequential therapy: Consists of a PPI and amoxicillin initially followed by a PPI, clarithromycin, and a nitroimidazole
- Current guidelines should be consulted for additional information.
Usual Adult Dose for Pelvic Inflammatory Disease
US CDC Recommendations: 500 mg orally twice a day for 14 days
Comments:
- Recommended as an optional component of IM/oral regimens for mild-to-moderately severe acute pelvic inflammatory disease (PID); regimens include ceftriaxone plus doxycycline (with or without this drug), cefoxitin/probenecid plus doxycycline (with or without this drug), or other parenteral third-generation cephalosporin plus doxycycline (with or without this drug).
- Since anaerobic organisms are suspected in the etiology of PID, the addition of this drug should be considered; it also effectively treats bacterial vaginosis, which is often associated with PID.
- Recommended to complete at least 14 days of therapy with doxycycline when a tubo-ovarian abscess is present; it provides more effective anaerobic coverage than doxycycline alone
- Current guidelines should be consulted for additional information.
Usual Adult Dose for Giardiasis
Some Experts Recommend: 250 mg orally 3 times a day for 5 to 7 days
Comments:
- Recommended as a regimen to treat giardiasis
- Current guidelines should be consulted for additional information.
Usual Adult Dose for STD Prophylaxis
US CDC Recommendations: 2 g orally once as a single dose
Comments:
- Ceftriaxone and azithromycin, are recommended as a regimen for sexually transmitted disease (STD) prophylaxis after sexual assault or abuse
- Current guidelines should be consulted for additional information.
Usual Adult Dose for Nongonococcal Urethritis
US CDC Recommendations: 2 g orally once as a single dose
Comments:
- In areas where T vaginalis is prevalent, recommended for recurrent or persistent urethritis in men who have sex with women
- Sexual partners should be referred for evaluation and appropriate treatment.
- Current guidelines should be consulted for additional information.
Usual Adult Dose for Balantidium coli
US CDC Recommendations: 500 to 750 mg orally 3 times a day for 5 days
Comments:
- Recommended as an alternative regimen for balantidiasis due to Balantidium coli
- Current guidelines should be consulted for additional information.
Usual Adult Dose for Dientamoeba fragilis
US CDC Recommendations: 500 to 750 mg orally 3 times a day for 10 days
Comments:
- Recommended as a regimen to treat infection due to Dientamoeba fragilis
- Current guidelines should be consulted for additional information.
Usual Pediatric Dose for Bacterial Infection
American Academy of Pediatrics (AAP) Recommendations
Neonates:
Loading dose: 15 mg/kg IV once as a single dose
Maintenance dose:
- Postmenstrual age up to 34 weeks: 7.5 mg/kg IV every 12 hours
- Postmenstrual age 35 to 40 weeks: 7.5 mg/kg IV every 8 hours
- Postmenstrual age greater than 40 weeks: 10 mg/kg IV every 8 hours
1 month or older:
- IV: 22.5 to 40 mg/kg/day IV in 3 or 4 divided doses
- Maximum dose: 4 g/day
- Oral: 15 to 50 mg/kg/day orally in 3 divided doses
- Maximum dose: 2.25 g/day
Comments:
Current guidelines should be consulted for additional information.
Usual Pediatric Dose for Amebiasis
35 to 50 mg/kg/day orally divided into 3 doses for 10 days
Comments:
Aspiration or drainage of pus is still needed for amebic liver abscess.
Uses: For the treatment of acute intestinal amebiasis (acute amebic dysentery) and amebic liver abscess
Usual Pediatric Dose for Pseudomembranous Colitis
AAP Recommendations:
- 1 month or older: 7.5 mg/kg orally (or IV) every 6 hours for 10 days
- Maximum dose: 500 mg/dose
IDSA and SHEA Recommendations for Children:
- An initial episode or first recurrence of nonsevere CDI: 7.5 mg/kg orally 3 or 4 times a day
- An initial episode of severe/fulminant CDI: 10 mg/kg IV 3 times a day
- Maximum dose: 500 mg/dose
- Duration of therapy: 10 days
Comments:
- AAP: Recommended for the first occurrence or first recurrence of mild to moderate CDI (oral formulation preferred)
- AAP: With vancomycin, recommended as IV therapy for the first occurrence of severe and complicated CDI
- Severe and complicated CDI may be characterized by intensive care unit admission, hypotension/shock, pseudomembranous colitis by endoscopy, ileus, or toxic megacolon.
- AAP: This drug should not be used beyond the first recurrence or for chronic therapy due to possible neurotoxicity.
- IDSA and SHEA: Recommended for the oral treatment of children with an initial episode or first recurrence of nonsevere CDI
- If this drug was used for the primary episode, vancomycin should be used for a first recurrence.
- IDSA and SHEA: For severe/fulminant CDI associated with critical illness, the addition of this IV drug to oral vancomycin may be considered.
- Current guidelines should be consulted for additional information.
Usual Pediatric Dose for intra-abdominal Infection
IDSA and SIS Recommendations: 30 to 40 mg/kg/day IV in divided doses every 8 hours
Comments:
- With other antibacterial agents, recommended as a combination regimen for empiric treatment of complicated intra-abdominal infection
- Current guidelines should be consulted for additional information.
Usual Pediatric Dose for Trichomoniasis
AAP Recommendations:
- Infants and children less than 45 kg: 45 mg/kg/day orally in 3 divided doses for 7 days
- Maximum dose: 2 g/day
- Children at least 45 kg and adolescents: 2 g orally once as a single dose
Comments:
- Recommended for prepubertal vaginitis (sexually transmitted infection [STI] related) due to T vaginalis in infants and children less than 45 kg
- Recommended for vulvovaginitis due to T vaginalis in children at least 45 kg and adolescents
- The patient’s sexual partner(s) should be treated simultaneously.
- Current guidelines should be consulted for additional information.
Usual Pediatric Dose for Nongonococcal Urethritis
AAP Recommendations:
Children at least 45 kg and adolescents: 2 g orally once as a single dose
Comments:
- In areas where T vaginalis is prevalent, recommended for recurrent or persistent urethritis in males who have sex with females
- Sexual partners should be referred for evaluation and appropriate treatment.
- Current guidelines should be consulted for additional information.
Usual Pediatric Dose for Bacterial Vaginosis
AAP Recommendations:
- Infants and children less than 45 kg: 15 to 25 mg/kg/day orally in 3 divided doses for 7 days
- Maximum dose: 2 g/day
- Children at least 45 kg and adolescent vulvovaginitis: 500 mg orally twice a day for 7 days
Comments:
- Recommended for prepubertal vaginitis (STI related) due to bacterial vaginosis in infants and children less than 45 kg
- Recommended for vulvovaginitis due to bacterial vaginosis in children at least 45 kg and adolescents
- All females with symptoms should be treated.
- Current guidelines should be consulted for additional information.
Usual Pediatric Dose for Giardiasis
- Some Experts Recommend: 5 mg/kg orally 3 times a day for 5 to 7 days
- Maximum dose: 250 mg/dose
Comments:
- Recommended as a regimen to treat giardiasis
- Current guidelines should be consulted for additional information.
Usual Pediatric Dose for STD Prophylaxis
AAP and US CDC Recommendations for Adolescents: 2 g orally once as a single dose
Comments:
Recommended in combination with ceftriaxone and azithromycin:
- AAP: As a regimen for prophylaxis after sexual victimization in postpubertal adolescents
- US CDC: As a regimen for STD prophylaxis after sexual assault or abuse
- Current guidelines should be consulted for additional information.
Usual Pediatric Dose for Balantidium coli
AAP and US CDC Recommendations: 35 to 50 mg/kg/day orally in 3 divided doses for 5 days
Maximum dose: 750 mg/dose
Comments:
- Recommended as an alternative regimen for balantidiasis due to B coli
- Current guidelines should be consulted for additional information.
Usual Pediatric Dose for Dientamoeba fragilis
AAP Recommendations: 35 to 50 mg/kg/day orally in 3 divided doses for 10 days
Maximum dose: 750 mg/dose
Comments:
- Recommended as a regimen to treat infection due to D fragilis
- Current guidelines should be consulted for additional information.
Usual Pediatric Dose for Pelvic Inflammatory Disease
US CDC Recommendations for Adolescents: 500 mg orally twice a day for 14 days
- Comments:
- Recommended as an optional component of IM/oral regimens for mild-to-moderately severe acute PID; regimens include ceftriaxone plus doxycycline (with or without this drug), cefoxitin/probenecid plus doxycycline (with or without this drug), or other parenteral third-generation cephalosporin plus doxycycline (with or without this drug).
- Since anaerobic organisms are suspected in the etiology of PID, the addition of this drug should be considered; it also effectively treats bacterial vaginosis, which is often associated with PID.
- Recommended to complete at least 14 days of therapy with doxycycline when the tubo-ovarian abscess is present, Pliva 334 with doxycycline is recommended for continued therapy; it provides more effective anaerobic coverage than doxycycline alone
- Current guidelines should be consulted for additional information.
Usual Pediatric Dose for Skin or Soft Tissue Infection
IDSA Recommendations:
1 month or older: 7.5 mg/kg IV every 6 hours
Maximum dose: 500 mg/dose
Comments:
- In combination with cefotaxime, recommended for the treatment of necrotizing infections of the skin, fascia, and muscle due to mixed infections
- Current guidelines should be consulted for additional information.
Usual Pediatric Dose for Skin and Structure Infection
IDSA Recommendations:
1 month or older: 7.5 mg/kg IV every 6 hours
Maximum dose: 500 mg/dose
Comments:
- In combination with cefotaxime, recommended for the treatment of necrotizing infections of the skin, fascia, and muscle due to mixed infections
- Current guidelines should be consulted for additional information.
Usual Pediatric Dose for Surgical Prophylaxis
IDSA, SHEA, ASHP, and SIS Recommendations:
Preoperative dose:
- Neonates less than 1.2 kg: 7.5 mg/kg IV as a single dose
- Neonates at least 1.2 kg and pediatric patients 1 month or older: 15 mg/kg IV as a single dose
Maximum dose: 500 mg/dose
Colorectal surgery prophylaxis: 15 mg/kg orally
Maximum dose: 1 g/dose
Comments:
- IV: This drug should be started within 60 minutes before the surgical incision.
- IV: A single prophylactic dose is usually sufficient; if prophylaxis is continued postoperatively, the duration should be less than 24 hours.
- IV: A single prophylactic dose is usually sufficient; if prophylaxis is continued postoperatively, the duration should be less than 24 hours.
- IV: Redosing may be needed for unusually long procedures.
- Oral: In most patients, mechanical bowel preparation combined with this oral drug plus oral neomycin should be administered in addition to IV prophylaxis; the oral regimen should be administered in 3 doses over about 10 hours, beginning after the mechanical bowel preparation, the afternoon and evening before the procedure.
- Coadministration with other agents may be recommended, depending on the type of procedure.
- Current guidelines should be consulted for additional information.
Uses: Recommended for surgical prophylaxis for the following procedures:
- Appendectomy for uncomplicated appendicitis
- Biliary tract: Open procedure; elective, high-risk laparoscopic procedure
- Colorectal
- Head and neck: Clean-contaminated cancer surgery; other clean-contaminated procedures (excluding tonsillectomy, and functional endoscopic sinus procedures)
- Hysterectomy: Vaginal or abdominal
- Small intestine: Obstructed
- Urologic: Clean-contaminated
Renal Dose Adjustments
Mild to moderate renal dysfunction: No adjustment is recommended.
Severe renal dysfunction or ESRD (not on hemodialysis): Data not available
Comments:
Accumulation of metabolites may occur in severe renal dysfunction or ESRD (not on hemodialysis); monitoring for drug-related side effects is recommended.
Liver Dose Adjustments
Mild to Moderate Liver Dysfunction: No adjustment recommended.
Severe Liver Dysfunction (Child-Pugh C):
- Immediate-release tablet, capsule, and IV formulations: The dose should be reduced by 50%.
- Extended-release tablets: Not recommended unless benefits are considered to outweigh risks.
Hepatic encephalopathy:
IV: Reduce dose as needed.
Comments:
Patients with mild to moderate liver dysfunction should be monitored for drug-related side effects.
Uses of PLIVA 334 pill
It is used to treat the following;
- Wounds
- Joint infections
- Amebiasis
- Skin ulcers
- Rosacea
- Bacteremia
- Bacterial infections and parasitic infections
- Aspiration pneumonia
- Mouth infections (including dental abscesses and infected gums)
- Reproductive system infections
- Infected insect bite
- Gastrointestinal (GI) tract infection
- Vaginosis
- Bed sores
- Infections of the bone, blood, joint, skin, heart, nervous system, lung, and other areas of the body
- Sexually transmitted diseases (STDs)
- Pelvic inflammatory disease.
Side effects of PLIVA 334 pill
Along with its needed effects, Pliva 334 may cause some unwanted effects. Although not all of these side effects may occur, if they do occur they may need medical attention.
Common side effects;
- Heartburn
- Spinning sensation
- Lightheadedness or dizziness
- Nausea
- Insomnia
- Distorted perception of self, surroundings, and movements
- Unusual bleeding or bruising
- Discharge from the vagina, vaginal irritation, or vaginal
- Weight loss
- Agitation
- Back pain and stomach (severe)
- Stomach cramps
- Sneezing
- Depression
- Fever
- Eye pain
- Drowsiness
- Painful, numbness, burning, or tingling sensation in the hands or feet
- Confusion
- Back pain
- Blurred, decreased vision or blindness
- Slurred or distorted speech patterns
- Dizziness
- Headache
- Irritability
- Hallucinations (hearing things or seeing that are not there)
- Unsteady or shaky walk pattern
- Problems with coordination or muscle control, and trembling of muscles
- Vomiting
- Weakness in the feet or legs, arms, and hands
- Tarry and black stools
- Bloody urine
- Lack of coordination
- Seizures
- Runny nose
- Stiff neck or back
- Painful or frequent urination
- Pelvic pressure
- Irregular heartbeat
- Ear congestion
- Clumsiness or unsteadiness
- Red spots on the skin
- Voice loss
- Chills
- Breathing difficulties
- Fainting
- Nasal congestion
- Skin rash, hives, redness, itching
- Burning or painful sensation while urinating
- Unusual fatigue
Some side effects of Pliva 334 may occur that usually do not need medical attention. These side effects may go away during treatment as your body adjusts to the medicine.
Also, your health care professional may be able to tell you about ways to prevent or reduce some of these side effects.
Uncommon side effects;
- Congestion
- Tender, swollen glands in the neck
- Trouble with swallowing
- Sharp or unpleasant metallic taste
- Distorted taste of food
- Cough
- Pain or tenderness around the eyes and cheekbones
- Constipation
- Dry mouth
- Dark urine
- Distorted vocalization while speaking
- Chest pain
- Fast heartbeat
- Bloating
- Sore throat
- Abdominal, side, or stomach pains possibly originating from the back
- Bleeding gums
- Swollen glands
- Ulcers and sores
- Yellow eyes or skin
- Difficult or painful urination
- Appetite loss
- White spots in the mouth or on the lips.
- Indigestion
Check with your doctor immediately if any of the following side effects persist or become severe while taking Pliva 334.
Side effects that the incidence is not known;
- Inability to have or keep an erection
- Pains during sexual intercourse
- Cloudy or bloody urine
- Chronic diarrhea
- Decreased interest in sexual intercourse
- Peeling, loosening, and blistering of the skin
- Lack of sexual desire, performance ability, or drive
- Increased urination of pale, dilute urine in large amounts
- Burning sensation while urinating
- Warmth sensation
- Chronic stomach pains
- Skin redness
- Redness of the neck, face, arms, and mostly, upper chest
- Loss of control over bladder
- Irritated and red eyes
- Red lesions of the skin, frequently with a purple center
- Muscle or joint pain
Check with your health care professional if any of the following side effects continue or are bothersome or if you have any questions about them:
General
The most serious side effects reported were neuropathy, optic, aseptic and peripheral neuropathy, encephalopathy, convulsive seizures, and meningitis.
Other side effects
- Common: Influenza-like symptoms and bacterial infection,
- Uncommon: Asthenia
- Rare: Fever/ pyrexia, flushing, and mucosal inflammation
- Incidence not reported: Overgrowth of Candida, malaise, face edema, peripheral edema, chest pain, chills, disulfiram-like reaction, weakness, and sensation of pelvic pressure.
Genitourinary
- Common: Moniliasis, dysmenorrhea, abnormal urine, vaginitis, urinary tract infection, and genital pruritus.
- Rare: Dark urine and chromaturia
- Incidence not reported: Cystitis, polyuria, vulva and vagina dryness, dysuria, dyspareunia, vaginal candidiasis, the proliferation of Candida in the vagina, pyuria, and incontinence.
Respiratory
- Common: Upper respiratory tract infection, rhinitis, sinusitis, pharyngitis
- Incidence not reported: Dyspnea, nasal congestion, hiccup.
Musculoskeletal
- Common: Myalgia
- Rare: Arthralgia
- Incidence not reported: Muscle spasms, fleeting joint pain, stiff neck
Hematologic
- Common: Leukopenia
- Rare: Neutropenia, thrombocytopenia, pancytopenia, agranulocytosis
- Incidence not reported: Eosinophilia, bone marrow aplasia, bone marrow depression, blood dyscrasia.
Dermatologic
- Rare: Stevens-Johnson syndrome, toxic epidermal necrolysis, angioedema, erythema multiforme, skin rash, pustular eruptions, acute generalized exanthematous pustulosis, pruritus
- Incidence not reported: Fixed drug eruption, swelling face, urticaria, hyperhidrosis, erythema, drug reaction with eosinophilia and systemic symptoms, erythematous rash, mild erythematous eruptions, pustulosis.
Hepatic
- Rare: Cholestatic hepatitis, increased liver enzymes (AST, ALT, alkaline phosphatase), cholestatic/mixed hepatitis, hepatocellular liver injury, jaundice, liver failure requiring a liver transplant, abnormal liver function tests
- Incidence not reported: Increased liver enzyme, hepatotoxicity/liver failure in patients with Cockayne syndrome, drug-induced hepatitis.
Hypersensitivity
- Rare: Anaphylaxis, anaphylactic shock, Jarisch-Herxheimer reaction
- Incidence not reported: Serum sickness-like reaction, immediate/delayed hypersensitivity reactions, anaphylactic reaction, and hypersensitivity.
Psychiatric
- Rare: Hallucinations
- Very rare: Psychotic disorders and confusion.
- Incidence not reported: Depression, insomnia, depressed mood, decreased libido, irritability, psychosis, disorientation, and psychotic reaction.
Ocular
- Rare: Optic neuropathy, diplopia, myopia, vision disorders (e.g., diplopia, myopia)
- Incidence not reported: Optic neuropathy/neuritis, saccadic eye movement, blurred vision, decreased visual acuity, changed color vision, light sensitivity.
Cardiovascular
Incidence not reported: Flattening of T-wave on ECG, tachycardia, palpitations, QT interval prolonged on ECG
Metabolic
Incidence not reported: Anorexia, decreased appetite.
Oncologic
An increased incidence of gastrointestinal and certain extraintestinal cancers have been reported in patients with Crohn’s disease. Breast and colon cancer have been reported in medical literature in Crohn’s disease patients treated with high doses of this drug for a prolonged duration; causality was not established
Incidence not reported: Breast cancer, colon cancer, gastrointestinal cancer, and certain extraintestinal cancers.
Local
Incidence not reported: Injection site reaction, thrombophlebitis.
Nervous system
- Common: Distorted taste/dysgeusia, dizziness, and headache.
- Rare: Convulsions, peripheral neuropathy, aseptic meningitis, Encephalopathy (e.g., paralysis, headache, incoordination, light sensitivity, confusion, stiff neck, subacute cerebellar drowsiness, hallucinations, fever, problems in movements and sight.), ataxia, somnolence, and seizures.
- Incidence not reported: Paresthesia, hearing problems peripheral sensory neuropathy, hypoesthesia, transient epileptiform seizures, dysarthria, tinnitus, distorted taste in mouth convulsive seizure, paralysis, numbness, vertigo, syncope, and nystagmus.
Gastrointestinal
- Common: Dry mouth, glossitis, abdominal pain, stomatitis, diarrhea, and nausea.
- Rare: Epigastralgia/ upper abdominal pain, vomiting, and pancreatitis.
- Incidence not reported: Dyspepsia, oral mucositis, the sudden growth of Candida in the mouth, proctitis, pseudomembranous colitis, tongue discoloration, furred tongue, mucositis, constipation, abdominal cramping, and gastrointestinal disturbances.
What if I miss a significant dose of PLIVA 334?
Nothing much will happen. Just that if you have missed significant doses of the drug, it will not be effective or potent enough to treat you for the ailment you took it for.
Do the following if you have missed a significant dose;
- If you forget to take one or more doses: take your next dose at the usual time and in the average amount. Do not take anymore than your doctor prescribed.
- If you miss one dose, skip it and continue with your normal schedule.
- Do not increase the dosage to catch up with the missed doses.
- You should consult your doctor on what to do if you don’t know what to do.
What if I overdose on PLIVA 334?
Seek immediate medical attention or call the Poison Help line at 1-800-222-1222.
Overdose symptoms may include nausea, vomiting, numbness, tingling, or problems with balance or muscle movement.
Warnings and precautions for taking PLIVA 334
Take note of the following precautions and warnings:
- If you have recently consumed alcohol or used disulfiram (Antabuse) during the last two weeks, you should not use Pliva 334.
- While using Pliva 334 and for at least 3 days after stopping it, avoid drinking alcohol or consuming foods or medications containing propylene glycol.
- Seizures and some other nervous system disorders have been documented in Pliva 334 patients. If you suffer any neurological symptoms such as headaches, seizures, vision abnormalities, weakness, numbness, or tingling, you should discontinue use immediately.
This medication does not treat viral infections like the common cold or flu.
This drug produced various forms of malignancies or tumors in animal experiments (mice and rats). It is unknown if these side effects would occur in anyone who used this medication. Inquire with your doctor about your risk.
You should not use this medication if you are allergic to Pliva 334 or tinidazole, or if you have any of the following conditions:
- You consumed alcohol during the last three days;
- In the last three days, you ingested foods or medications containing propylene glycol
- Disulfiram (Antabuse) was taken during the last 14 days.
Pliva 334 may be harmful to an unborn child. Pliva 334 should not be used for trichomoniasis treatment during the first pregnancy trimester. If you get pregnant, notify your doctor.
Pliva 334 is not authorized for all usage in children and teens. Pliva 334 is not recommended for the treatment of infections of the vagina in females who have not yet started menstruating.
To ensure that you can take this medication safely, inform your doctor if you have had:
- A disease of the liver;
- Kidney disease (or being on dialysis);
- A cardiac rhythm problem;
- Crohn’s disease (a stomach or intestinal illness).
- Anemia (lack of red blood cells) or low white blood cell (WBC) counts
- A fungal infection in any part of your body
- A nerve condition
Pliva 334 has been linked to cancer in animal studies. It is unknown if this would happen to people. Inquire with your doctor about your risk.
Breastfeeding should be avoided for 24 hours after taking Pliva 334. If you are using a breast pump at this period, discard the milk and do not give it to your baby.
Drug Interactions of PLIVA 334
Sometimes it is not safe to use certain medicines at the same time. Some drugs can affect the blood levels of other drugs you use, which may increase side effects or make the medicines less effective.
Tell your doctor about all your current medicines. Many drugs can affect Pliva 334, especially:
- An antidepressant
- Asthma medication
- Busulfan or other cancer medicine
- Heart or blood pressure medication
- Lithium or other antipsychotic medicine
- Medicine to treat malaria, HIV, or other infection
- A blood thinner – warfarin, Coumadin, Jantoven.
Pliva 334 should not be used with any of the following drugs or medications:
- Disulfiram
- Alcohol or alcoholic product
- Pimozide
- Cisapride
- Thioridazine
- Dronedarone
Pliva 334 may also interact with the following drugs or medications:
- Quinidine
- Fluorouracil
- Phenobarbital
- Amiodarone
- Carbamazepine
- Birth control pills
- Tacrolimus
- Cyclosporine
- Vecuronium
- Drugs that elongate the interval of QT (causing abnormal heart rhythms) like ziprasidone and dofetilide.
- Phenytoin
- Cimetidine
Many other drugs may interact with Pliva 334. This includes over-the-counter drugs, herbal products, and vitamins.
IS PLIVA 334 safe?
Yeah, it is, only when taken as prescribed. To get the most out of it, take it exactly as directed. In general, you should take the lowest effective dosage for the shortest time.
Always make sure to consult your doctor before taking medications like this because there are certain conditions you might be in that taking Pliva 334 will be very harmful to you.
This will make it easier for you to go about your regular routines and have a better, more active quality of life.
IS PLIVA 334 a controlled drug?
It is not a controlled substance under the Controlled Substances Act (CSA).
IS PLIVA 334 available in generic versions?
Pliva 334 is the generic version of Pliva 334.
Alternatives or substitutes to PLIVA 334
- Clindamycin
- MetroGel-Vaginal
- MetroCream
- MetroGel
- MetroLotion
- Noritate,
- Nuvessa
- Rosaclear,
- Rosadan
- Vandazole
- Flagyl
What is the price of PLIVA 334?
The cost for a pliva 334 oral tablet 250 mg is around $11 for a supply of 6 tablets, depending on the pharmacy you visit. Prices are for cash-paying customers only and are not valid with insurance plans.
Pricing depends on what form or strength of Pliva 334 you are purchasing, and also the pharmacy you are buying the drug from.
You can also check drugs.com, for a standard pricing guide for any form or dose/strength you want on Pliva 334.
Where can I buy PLIVA 334?
Since it’s only available only by a doctor’s prescription, it can only be bought at your local pharmacy and hospitals.
How do I store PLIVA 334?
- Capsules: Store at 15-25`C (59-77`F).
- IV solution (in single-dose plastic containers): Store in moisture barrier wrap at 20 to 25`C (68F to 77F) and protect from light during storage; do not refrigerate.
- Tablets: Store below 25C (77F); protect from light.
Store Pliva 334 at room temperature away from light and moisture. Do not store it in the bathroom. Keep all medications away from children and pets.
Do not flush medications down the toilet or pour them into a drain unless instructed to do so. Properly discard this product when it is expired or no longer needed. Consult your pharmacist or local waste disposal company.
Final thoughts on PLIVA 334 by Healthsoothe
Keep all appointments with your doctor and the laboratory. Your doctor will order certain lab tests to check your response to Pliva 334.
Before having any laboratory test, tell your doctor and the laboratory personnel that you are taking Pliva 334.
Do not let anyone else take your medication. Your prescription is probably not refillable. If you still have symptoms of infection after you finish the Pliva 334, call your doctor.
It is important for you to keep a written list of all of the prescription and nonprescription (over-the-counter) medicines you are taking, as well as any products such as vitamins, minerals, or other dietary supplements.
You should bring this list with you each time you visit a doctor or if you are admitted to a hospital. It is also important information to carry with you in case of emergencies.
Pliva 334, contained in Pliva 334 has been shown to be carcinogenic in mice and rats. Unnecessary use of the drug should be avoided. Its use should be reserved only for conditions for which it is approved.
While taking Pliva 334 and for 3 days after your last dose, do not drink alcohol or consume foods, medicines, or other products that contain alcohol or propylene glycol.
You may have unpleasant effects such as headaches, nausea, vomiting, stomach cramps, and warmth or tingling under your skin.
Do not use Pliva 334 to treat any condition that has not been checked by your doctor because using antibiotics like Pliva 334 when they are not needed increases your risk of getting an infection later that resists antibiotic treatment.
The information provided here below is mostly for doctors, physicians, and pharmacists who are considering prescription WES 303 for their patients.
General:
Without compatibility studies, Pliva 334 must not be mixed with other drugs.
In mixed aerobic and anaerobic infections, this drug should be used with antimicrobials appropriate for the treatment of aerobic infection.
To reduce the development of drug-resistant organisms and maintain effective therapy, antibiotics should be used only to treat or prevent infections proven or strongly suspected to be caused by susceptible bacteria.
Culture and susceptibility information should be considered when selecting/modifying antibacterial therapy or, if no data are available, local epidemiology and susceptibility patterns may be considered when selecting empiric therapy.
Monitoring:
- General: Drug-related side effects in elderly patients, patients with severe renal dysfunction or ESRD not undergoing hemodialysis, peritoneal dialysis patients, and patients with mild to moderate liver dysfunction
- Hematologic: Total and differential leukocyte counts (before and after oral therapy); CBC (before, during, and after prolonged/repeated IV therapy); CBC in patients with blood dyscrasias (with IV therapy)
- Hepatic: Liver function tests in patients with Cockayne syndrome (before starting, within 2 to 3 days after starting, frequently during, and after the end of therapy)
Patient advice:
- Avoid missing doses and complete the entire course of therapy.
- Discontinue the use of alcoholic beverages or products containing propylene glycol while taking this drug and for at least 3 days after discontinuing use; abdominal cramps, nausea, vomiting, headaches, and flushing may occur.
- Report neurologic symptoms that occur during therapy.
- Patients with Cockayne syndrome: Stop this drug at once and contact the healthcare provider if any symptoms of potential liver injury (eg; abdominal pain, nausea, change in stool color, jaundice) occur.
All right, guys, that is it for now for pliva 334. I hope Healthsoothe answered any questions you had concerning pliva 334.
Feel free to contact us at [email protected] if you have further questions to ask or if there’s anything you want to contribute or correct to this article. And don’t worry, Healthsoothe doesn’t bite.
You can always check our FAQs section below to know more about pliva 334.
And always remember that Healthsoothe is one of the best health sites out there that genuinely cares for you.
Frequently Asked Questions about Pliva 334
What happens if you drink alcohol with pliva 334?
Drinking alcohol while taking Pliva 334 is not recommended because the combination of Pliva 334 and alcohol can cause a reaction (often referred to as a disulfiram-like reaction) in some people.
Symptoms may include flushing, headaches, nausea, vomiting, and stomach cramps. There has been one reported death associated with this reaction.
The product information and health professionals recommend not drinking alcohol during Pliva 334 treatment and for 3 days after finishing the course.
However, there is controversy around this reaction because while some studies have shown serious problems for some people taking Pliva 334, others have shown the combination does not cause any problems.
Large clinical trials in humans have never been conducted to confirm this interaction. The reaction has been referred to as a disulfiram-like reaction – disulfiram is a medication given to people to discourage alcohol consumption.
When a person consumes alcohol, the body breaks it down in two steps. First, it breaks alcohol down into a compound called acetaldehyde.
Acetaldehyde is responsible for the unwanted effects of alcohol such as nausea, vomiting, and flushing, and is toxic. Next, the body reduces acetaldehyde to acetate using an enzyme called aldehyde dehydrogenase.
Acetate is easily oxidized by our body into carbon dioxide (CO2), which we then breathe out.
Disulfiram blocks the effects of this enzyme which leads to acetaldehyde accumulation causing symptoms such as skin redness, palpitations, nausea, vomiting, headache, and in severe cases a dangerous rapid heart rate or a sudden drop in blood pressure.
It was thought that Pliva 334 blocked the enzyme aldehyde dehydrogenase as well, although this now seems to be incorrect.
Several studies that have investigated the reaction of Pliva 334 with alcohol have found evidence of the existence of this interaction to be absent or weak. It does seem that the concern attached to this reaction is overstated.
It is possible that the reaction could just be a side effect of Pliva 334 or potentially only occur in a small sub-group of susceptible people because the reaction does not appear to occur in everybody.
There needs to be more research done investigating this potential interaction, but because doctors are unable to say which people are more at risk of this interaction, it is best to err on the side of caution and avoid alcohol while taking Pliva 334 until more is known.
How long does it take for pliva 334 to work?
Pliva 334 starts to work 1 to 2 hours after you take it, because it is quickly absorbed, and it reaches its maximum concentration after 20 minutes to 3 hours.
But it may take a couple of days before you start to feel better or notice an improvement in your symptoms.
It is important that you still finish the course of Pliva 334 that your doctor has prescribed, even if you feel better, because the infection may still be present, and it could flare up or recur if you haven’t finished the course.
How long does it take pliva 334 to leave your system?
It will take about 44 hours for Pliva 334 to be cleared from your system.
The elimination half-life of Pliva 334 is approximately 8 hours. Therefore it will take about 44 hours for it to be cleared from your system.
Does pliva 334 treat chlamydia?
Pliva 334 does not treat chlamydia and is not a recommended treatment for chlamydia, but it may be given if symptoms of chlamydia persist after finishing a course of first-line treatments for chlamydia such as doxycycline, azithromycin, or levofloxacin.
When Pliva 334 is given as follow-up treatment for persistent symptoms of chlamydia, usually in addition to other antibiotics such as erythromycin, it is there to provide treatment for other possible bacterial causes that may cause similar symptoms to chlamydia.
Infections linked with sexual activity that is usually treated with Pliva 334 include bacterial vaginosis, trichomoniasis, and moderate-to-severe pelvic inflammatory disease.
Can Pliva 334 cause a yeast infection?
About 10% of women report a vaginal yeast infection (vaginal candidiasis) as a side effect of Pliva 334 treatment.
This is because Pliva 334 not only kills the bacteria responsible for conditions such as bacterial vaginosis but useful bacteria in the vaginal flora that help to keep other microbes in check.
This can result in an overgrowth of the candida yeast. Symptoms of a yeast infection include itching and a thick, white odor-free discharge and it can be easily treated with medication such as fluconazole.
Oral yeast infections (oral thrush or oral candidiasis) have also been reported with Pliva 334 oral treatment.
Symptoms may include creamy white lesions on the tongue, inner cheeks, or sometimes on the roof of your mouth, gums, and tonsils; mouth redness, burning, or soreness, and a furry or swollen tongue.
Treatment is with an antifungal mouthwash such as nystatin or oral antifungal tablets or capsules.
Can you have sex while taking Pliva 334?
If you are taking oral Pliva 334 or using Pliva 334 gel for an infection that is linked with sexual activity, such as trichomoniasis, pelvic inflammatory disease, or bacterial vaginosis you should not have sex for 7 days after single-dose therapy (or until you have completed the 7-day treatment regimen) and your symptoms have resolved.
This will help reduce the risk of reinfection. If your sexual partners are also being treated, then abstain from sexual intercourse until they have finished treatment.
This means you should abstain from all forms of sex (including oral sex, vaginal sex, anal sex, and sex with a condom).
This advice even applies to women being treated for bacterial vaginosis, which although not sexually transmitted, is linked with sexual activity.
Researchers think that sex may change the bacterial environment in your vagina, making bacterial overgrowth more likely. Abstaining from sex during treatment gives the vaginal flora time to return to normal.
If you are taking Pliva 334 for other reasons, such as for an abdominal, bone, heart, lung, or skin infection, you do not need to abstain from sex.
Can you use Soolantra and Pliva 334 together?
Yes, you can use both Soolantra cream and Pliva 334 gel or cream together as there is no clinical interaction, but usually, you would only use one or the other.
Both Soolantra cream and Pliva 334 cream or gel are used to treat the inflammatory bumps and pimples that are symptoms of rosacea (papulopustular rosacea).
They are both applied to the skin once daily. It is currently not known how either medicine works to treat rosacea.
Both Soolantra and Pliva 334 applications are not usually prescribed together as:
- It may increase the risk of localized side effects
- If the treatment works you won’t know which product helped and you may be using one unnecessarily.
When diagnosed with rosacea that has inflammatory bumps and pimples, your healthcare professional may prescribe a number of options which may or may not include either Soolantra cream or Pliva 334 gel or cream.